Bioprinted model of glioblastoma
Assessing the effect of extracellular matrix composition on brain tumor resistance in a bioprinted model of glioblastoma
Glioblastoma multiforme (GBM) is the most common primary brain cancer and of the most lethal with almost certain patient death within two years of diagnosis1. These tumors can escape the natural host immune surveillance and subvert the physiological role of multiple brain cell types including vascular endothelial cells of the blood-brain barrier (BBB) and immune cells2,3. Standard therapy for GBM consists of surgical resection of the main tumoral mass followed by radiation and chemotherapy4. Except for a few exceptions, tumors initially respond to these treatments, however, no current regimen can overcome tumor recurrence after which the chances of patient survival drop drastically to 6 months. While the exact mechanisms underpinning drug resistance are still largely unclear, the current leading hypothesis is that a small percentage of cancer cells with self-renewal potential, also named glioblastoma stem cells (GSC)5, could survive and be selected during the therapy. Such a limited population of resistant GSCs may eventually spread undetected through the brain and regenerate the tumoral mass during recurrences. Targeting GSCs and their specific niches represents a promising strategy for curing GBM, however, the field lacks robust translational models able to capture the complex set of molecular and biomechanical cues that are thought to support GSCs and that are normally generated by the healthy cells of the brain.
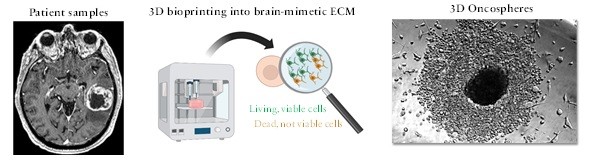
In our lab, we have recently established an enabling 3D model of GBM designed to recapitulate peculiar aspects of the brain tumor microenvironment that are known to modulate cancer resistance in vivo and including tissue-specific components of the extracellular matrix as well as human brain cells. This project will consist in conducting a proof-of-concept study to assess drug resistance of patient-derived tumors against existing and novel therapies. We will test the hypothesis that our new in vitro system supports patient-derived GSCs’ survival and maintenance of self-renewal markers when cancer cells are exposed to therapeutics used in the clinic. The project will include training in the use of advanced bioprinting methods, cell culture, and microscopy imaging.
Rodents have been used for decades to obtain a better understanding of molecular mechanisms underpinning cancer cell resistance, however, the results of animal studies do not always translate in humans as demonstrated by many failed clinical trials of the past decade (doi: 10.1158/1078-0432.CCR-21-2750). In vitro studies have provided new insights into the mechanisms of cancer resistance6; however, existing models do not recapitulate the native tumor microenvironment of GBM.
Recent studies have demonstrated that the ability of GSCs of surviving and proliferating in vitro requires molecular and biomechanical cues that are normally generated by the healthy cells of the brain, including its peculiar extracellular matrix mostly consisting of hyaluronic acid and low levels of collagens.
More advanced models are urgently needed for maintaining the GSCs viable in vitro and for capturing the complex heterogeneous nature of this lethal tumor.
References:
1. Lin, D. et al. Trends in Intracranial Glioma Incidence and Mortality in the United States, 1975-2018. Front. Oncol. 11, 748061 (2021).
2. Pacioni, S. et al. Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood–Brain Barrier. Cancers 12, 18 (2019).
3. Himes, B. T. et al. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 11, 770561 (2021).
4. Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
5. Gilbertson, R. J. & Rich, J. N. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer 7, 733–736 (2007).
6. Dogan, E. et al. Cancer Stem Cells in Tumor Modeling: Challenges and Future Directions. Adv. NanoBiomed Res. 1, 2100017 (2021).
Director

Riccardo Barrile
Assistant Professor, CEAS - Biomedical Eng
220 UCBIOSCI